Among these are:
- High chemical purity and resistance
- High softening temperature and thermal resistance
- Low thermal expansion with high resistance to thermal shocks
- High transparency from the ultraviolet to the infrared spectral range
- High radiation resistance
About SiO2 and other glass types
Silicon Dioxide – Glass – Quartz – Fused Silica
Silicon Dioxide (SiO2) is the simplest chemical composition of glass. Quartz is the most stable crystal modification at normal temperature and pressure conditions. Quartz is one of the most common minerals in the earth's crust. Glass (from “glasa”, Germanic for amber, the shiny or shimmery) also consists of silicon and oxide, but is a uniform amorphous solid material. Many glass varieties are clear and transparent respectively. This means transmissibility for the visible spectrum of light. In general, such material is associated with the term glass. Transparent materials allow light to pass through them without diffusing (scattering) the light.
Most common types of glass
At least 2000 years ago humans learned how to lower the glass softening temperatures by adding lime and soda before heating, which resulted in a glass containing sodium and calcium oxide.
Glass – Additives and the industrial use of glass
The use of glass as one of the oldest, but also very important materials for the industry is linked with the application of additives. Chemicals like soda (sodium carbonate, Na2CO3) and in the past also potash (potassium carbonate, K2CO3), manganese oxide and metal oxides influence the properties of glass. Manufactured glass is a material formed by heating a mixture of sand, soda and lime to a high temperature and stays in a molten, liquid state. Glass can be made from pure silica, but quartz glass (also referred as quartz) has a high glass transition point at around 1200°C, which makes it difficult to form into panes or bottles.
Quartz glass is the purest form of SiO2 and therefore the most valuable and sophisticated variety. Extremely clear glass can be used for optical fibers. Therefore synthetic quartz glass is used to transmit light across many kilometers. Many glasses block ultraviolet radiation, but only pure fused silica (only SiO2) is transparent for wavelengths < 350 nm (UV). Quartz glass also exists as an opaque variety and with different coloration to change the physical and chemical properties like transmission or absorption for specific wavelengths (filter glass). The opaque material at Heraeus, OM® 100, is also used as a heat barrier or for diffuse scattering of IR radiation.
Raw materials
At first glance, quartz glass appears very simple, both chemically and structurally, since it is made from a single oxide component (silicon dioxide – SiO2).
Chemical structure
Silica, as it is also known, is found throughout the earth’s crust. However, only a small fraction has sufficient purity (> 99.98 %) to be suitable as raw material for quartz glass. Sand at the beach is also SiO2, but isn’t suitable for use in the semiconductor industry.
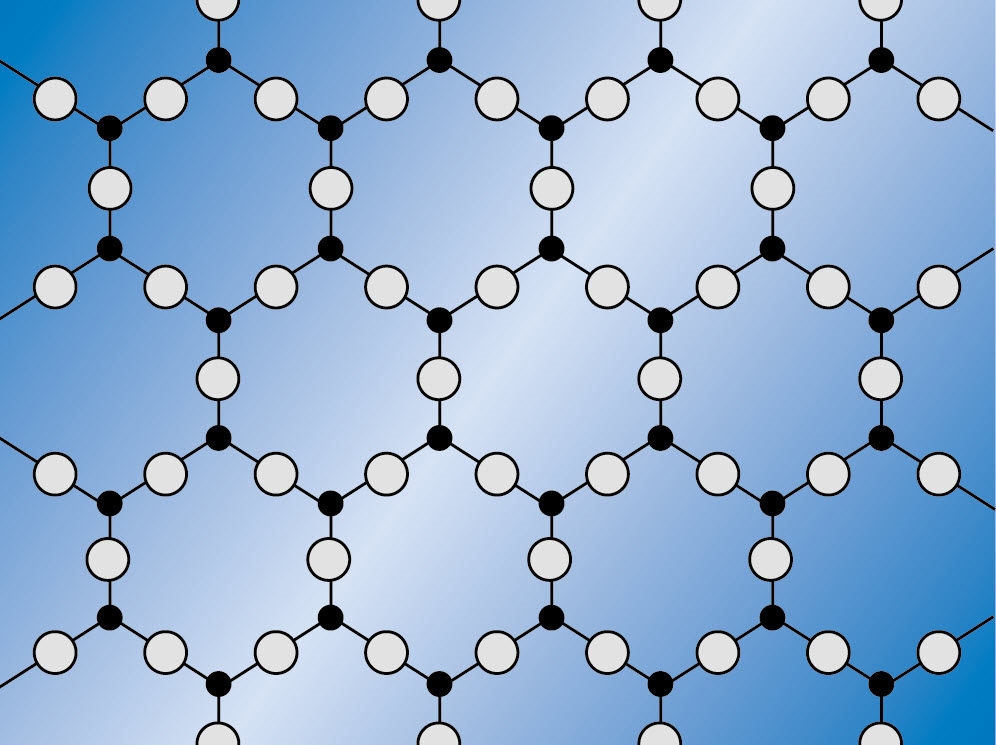
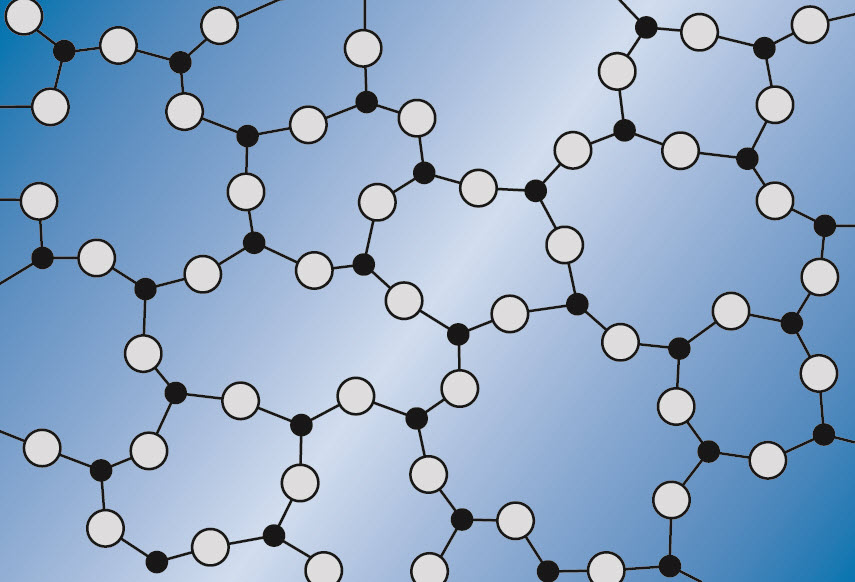
Structure of quartz and fused silica
In the quartz glass structure all atoms are bonded to at least two others. The strength of the silicon-oxygen (Si-O) chemical bond gives quartz glass high temperature stability and chemical resistance. But the structure is also rather open with wide spaces (interstices) between the structural units. This accounts for higher gas permeability and a much lower thermal expansion coefficient for quartz glass relative to other materials.
Crystalline SiO2 structure
Glassy SiO2 structure
Chemical purity
Purity is crucial for most industrial applications and processes. Fused silica has an outstandingly high purity and therefore is indispensable in the fabrication of high-tech products.
Despite existing at very low levels, contaminants have subtle yet significant effects. Purity is mostly determined by the raw material, the manufacturing method and subsequent handling procedures. Special precautions must be taken at all stages of manufacture to maintain high purity. Additionally, Heraeus uses different purification steps to improve the quality of the quartz sand as raw material even further.
The most common impurities are metals (such as Al, Na and Fe among others), water (present as OH groups) and chlorine. These contaminants not only affect the viscosity, optical absorption and electrical properties of the quartz glass but they also influence the properties of material processed in contact with the quartz glass during the final use application.
The purities of fused quartz and fused silica are outstandingly high. Synthetic fused silica from Heraeus contains total metallic contamination below 1ppm. For fused quartz the amount is approximately 20 ppm and consists primarily of Al2O3 with much smaller amounts of alkalis, Fe2O3, TiO2, MgO and ZrO2.

OH content
In addition to metallic impurities, fused quartz and fused silica also contain water present as OH units. OH content influences the physical properties like attenuation and viscosity. Generally, high OH content means lower use temperature. Typical values are given in the table. Electrically fused quartz has the lowest hydroxyl content (< 1 – 30 ppm) since it is normally made in vacuum or a dry atmosphere. Hydroxyl content in this range is not fixed in the glass structure. It can go up or down depending on the thermal treatment and amount of moisture the quartz glass is exposed to at elevated temperature. Flame fused quartz has significantly more hydroxyl (150 – 200 ppm) since fusion occurs in a hydrogen/ oxygen flame. Due to the production method, synthetic fused silica has similar high OH contents of up to 1000 ppm.
Chemical behavior
Synthetic silica produced by flame hydrolysis of silicon tetrachloride can have high (> 1000 ppm) or very low hydroxyl content depending on whether a hot chlorination step is employed to remove it. The hydroxyl level can be so high because the silica particles that result from the hydrolysis reaction are extremely fine and therefore have tremendous surface area capable of absorbing moisture present in the flame.
The main attributes of electrically fused materials are the low hydroxyl content and reduced devitrification rates. The low hydroxyl content increases infrared transparency and viscosity. The higher viscosity results in an increased maximum use temperature as well as helping to inhibit devitrification. Devitrification is also restrained by the neutral/ reducing atmosphere used during melting. This causes the material to be slightly oxygen deficient, which helps to restrain devitrification.
The high resistance of quartz glass to elements and compounds is another advantage for high-end applications. Fused quartz is outstandingly resistant to water, salt solutions and acids. Only hydrofluoric and phosphoric acid can attack it. Metals which are free from oxide, with the exception of alkalis and alkaline-earths, do not react with fused quartz or fused silica.
Quartz glass is sensitive to all alkali and alkaline-earth compounds because even slight traces hasten devitrification at high temperatures. It is always advisable to remove fingerprints, which contain traces of alkalis, from quartz glass with alcohol before heating.
Thermal properties
One of the most attractive features of quartz glass is its very low thermal coefficient of expansion (CTE). The average CTE value for quartz glass at about 5.0 × 10 -7/ °C is many times lower than that of other common materials. To put this in perspective, imagine if 1 m3 blocks of stainless steel, borosilicate glass and quartz were placed in a furnace and heated by 500 °C. The volume of the stainless steel block would increase by more than 28 liters and that of the borosilicate block by 5 liters. The quartz block would expand by less than one liter. Such low expansion makes it possible for the material to withstand very severe thermal shock.
It is possible to rapidly quench thin particles of quartz glass from over 1000 °C by plunging them into cold water without breakage. However, it is important to realize that the thermal shock resistance depends on factors other than CTE such as surface condition (which defines strength) and geometry. The various types of fused silica and fused quartz have nearly identical CTE’s and thus can be joined together with no added risk of thermally induced breakage.
Technical properties
Electrically Fused Quartz
Flame Fused Quartz
Fused Silica
Thermal data
Softening temperature (°C)
Annealing temperature (°C)
Strain temperature (°C)
Max. working temperature continuous (°C)
Max. working temperature short-term (°C)1710
1220
1125
1160
13001660
1160
1070
1110
12501600
1100
1000
950
1200Mean specific heat
(J/kg · K)0 ... 100 °C
0 ... 500 °C
0 ... 900 °C772
964
1052772
964
1052772
964
1052Heat conductivity
(W/m · K)20 °C
100 °C
200 °C
300 °C
400 °C
950 °C1.38
1.47
1.55
1.67
1.84
2.681.38
1.47
1.55
1.67
1.84
2.681.38
1.47
1.55
1.67
1.84
2.68Mean expansion coefficient
(K–1)0 ... 100 °C
0 ... 200 °C
0 ... 300 °C
0 ... 600 °C
0 ... 900 °C
– 50 ... 0 °C5.1 × 10–7
5.8 × 10–7
5.9 × 10–7
5.4 × 10–7
4.8 × 10–7
2.7 × 10–75.1 × 10-7
5.8 × 10–7
5.9 × 10–7
5.4 × 10–7
4.8 × 10–7
2.7 × 10–75.1 × 10–7
5.8 × 10–7
5.9 × 10–7
5.4 × 10–7
4.8 × 10–7
2.7 × 10–7Mechanical properties
Mechanical properties, strength and reliability
The theoretical tensile strength of silica glass is greater than 1 million psi. Unfortunately, the strength observed in practice is always far below this value. The reason is that the practical strength of glass is extrinsically determined rather than being solely a result of an intrinsic property like density. It is the surface quality in combination with design considerations and chemical effects of the atmosphere (water vapor in particular) that ultimately determine the strength and reliability of a finished piece of quartz glass. Because of stress concentration on surface flaws, failure most always occurs in tension rather than compression.
In other words: „reliablility depends on the chance“.
This could also be stated as the probability that the piece will experience a mechanical stress greater than the strength of any existing flaws. As a result of this dependence on probability, reliability decreases as the size of the glass article increases. Similarly, if the number of pieces in service increases, so does the chance of experience a failure.
Surface condition is very important. For example, machined surfaces tend to be weaker than fire polished ones. Also, older surfaces are usually weaker than younger ones due to exposure to dust, moisture or general wear and tear. These factors have to be considered thoroughly when comparing the strengths of different “brands” of quartz glass.
This is because these tests in reality often turn out to be just comparisons of surface quality resulting from sample preparation, small differences in which easily overwhelm any differences in intrinsic strength.
Mechanical Data
Electrically Fused Quartz
Flame Fused Quartz
Fused Silica
Density (g/cm3)
2.203
2.203
2.201
Mohs hardness
5.5 ... 6.5
5.5 ... 6.5
5.5 ... 6.5
Micro hardness (N/mm2)
8600 ... 9800
8600 ... 9800
8600 ... 9800
Knoop hardness (N/mm2)
5800 ... 6100
5800 ... 6100
5800... 6200
Modulus of elasticity at 20 °C (N/mm2)
7.25 × 104
7.25 × 104
7.25 × 104
Modulus of torsion (N/mm2)
3.0 × 104
3.1 × 104
3.0 x 104
Poisson’s ratio
0.17
0.17
0.17
Compressive strength (approx.) (N/mm2)
1150
1150
1150
Tensile strength (approx.) (N/mm2)
50
50
50
Bending strength (approx.) (N/mm2)
67
67
67
Torsional strength (approx.) (N/mm2)
30
30
30
Sound velocity (m/s)
5720
5720
5720
Optical properties
The optical properties of fused silica offer striking opportunities for research and industry: the broad transparent transmission range covers the complete visible spectrum and extends far into the infrared and ultraviolet regions. Transmission can be influenced by material purity and the method of manufacture. Additionally transmission ranges can be customized to meet your application by adding doping materials.
The intrinsic UV and IR absorption edges in silica glass are located at roughly 0.180 and 3.5 microns wavelengths, respectively. The intrinsic UV absorption edge results from the onset of electronic transitions with Si-O network at the point where photon energy exceeds the network bandgap energy. The intrinsic IR edge arises due to lattice (multi-phonon) vibrations of the Si-O network.
Various overtones of the fundamental SiO4 tetrahedron vibrational modes are the first to be observed. These intrinsic absorption edges are then further modified by the presence of impurities. Metallic impurities shift UV edge to higher wavelengths. Water (OH) introduces absorption bands just below the IR edge. The strongest of these is the fundamental O-H stretching band at 2.73 microns.
Electrical properties
Controlled heat management and sustained high temperatures is crucial in many industrial processes, especially in semiconductor industries.
Fused silica is a good electrical insulator, retaining high resistivity at elevated temperatures and excellent high-frequency characteristics. The large band gap inherent in the electronic structure of the silicon-oxygen bond results in electrical conduction being limited to current carried by mobile ionic impurities. Since the level of these impurities is very low, the electrical resistivity is correspondingly high.
Since ionic conduction is related to the diffusion coefficient of the ionic carriers, the resistivity also has a strong exponential temperature dependence. Hence, unlike typical conductors such as metals, the resistivity decreases with increasing temperature.
The dielectric constant of quartz glass has a value of about 4 which is significantly lower than that of other glasses. This value changes little over a wide range of frequencies. The reason for the low dielectric constant is, once again, the lack of highly charged mobile ions but it also results from the stiffness of the silicon-oxygen network which imparts a very low polarizability to the structure.
Parameter
Electrically Fused Quartz
Flame Fused Quartz
Fused Silica
Electrical resistivity in Ω × m
20 °C
400 °C
800 °C
1200 °C1018
1010
6.3 × 106
1.3 × 1051018
1010
6.3 × 106
1.3 × 1051016
1010
6.3 × 106
1.3 × 105Dielectric strength in KV/mm
(sample thickness ≥ 5 mm)20 °C
500 °C25 ... 40
4 ... 525 ... 40
4 ... 525 ... 40
4 ... 5Dielectric loss angle (tg δ)
1 kHZ
1 MHz
30 GHz5.0 × 10 –4
1.0 × 10 –4
4.0 × 10 –45.0 × 10 –4
1.0 × 10 –4
4.0 × 10 –45.0 × 10 –4
1.0 × 10 –4
4.0 × 10 –4Dielectric constant (ε)
20 °C: 0 ... 106 Hz
23 °C: 9 × 108 Hz
23 °C: 3 × 1010 Hz3.70
3.77
3.813.70
3.77
3.813.70
3.77
3.81Light induced damage
Because it has very low absorption down into the vacuum ultraviolet spectral range (the cutoff is at about 160 nm for a 1 mm thickness), synthetic fused silica is used for optical lenses in high-energy laser applications and as envelope material for ultraviolet light sources such as excimer or deuterium lamps. Depending on the exact experimental conditions, such as the wavelength, energy density and peak intensities in pulsed laser applications, various kinds of damage can occur to the glass.
At very high laser intensities, photoionization and plasma generation can take place locally at certain positions in the glass. This mechanical damage typically appears at the front or rear surface of the optical element. A related type of mechanical damage which can occur is the generation of fine microchannels within the glass along the propagation direction of a laser beam.
In addition to these visible damage phenomena, a more subtle damage mechanism can take place as defect centers (sometime called color centers) are generated in a photochemical process under irradiation of the glass. These centers cause absorption at characteristic wavelengths. Examples are the E’ center with an absorption maximum at 215 nm and the non-bridging oxygen hole (NBOH) center at 265 nm. The NBOH hole also emits red fluorescence at about 650 nm when excited in its absorption band. These defects also interact with dissolved hydrogen in the glass. Hydrogen can passivate E’ centers to create SiH groups, and NBOH centers to create SiOH groups, thus mitigating the transmission loss at the absorption wavelengths of these defects. Therefore, the hydrogen concentration is often controlled precisely during production processes, and measured by Raman spectroscopy in the analytical lab.
A third type of damage which can take place is a change in the refractive index of the silica glass due to restructuring of the glass network under irradiation. The refractive index can either increase (called compaction), or it can decrease (rarefaction), depending on the silica glass type and the irradiation conditions.

